This chapter demonstrates the spontaneous formation of the much
contested ferryl ion, Fe
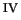
O

, in an aqueous solution
of iron(II) and hydrogen peroxide by means of first principles molecular
dynamics simulations. Starting from hydrogen peroxide coordinated
to pentaaqua iron(II) in water, we show that the oxygen-oxygen bond
breaks spontaneously to form [(H

O)
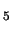
Fe
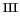
OH]

and a
very short living OH. radical. This radical abstracts immediately
a hydrogen from a water ligand to form
[(H

O)
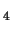
Fe
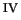
(OH)

]

and a water molecule.
The hydrated ferryl ion [(H

O)
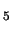
Fe
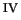
O]

is formed
in a second step by proton donation from one
OH ligand to the solvent. Starting from separated hydrogen peroxide
and pentaaqua iron(II) in water, we find a reactive pathway in
which the ferryl ion is formed in a more direct way. As soon as
H

O

enters the iron(II) coordination shell, the
oxygen-oxygen bond breaks and again an OH ligand and a short
living OH. radical is formed. The radical abstracts the hydrogen
from the OH ligand to form again the ferryl ion.