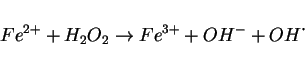 |
(59) |
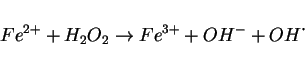 |
(59) |
The other mechanisms involve the formation of a
highly reactive iron-oxo complex such as the ferryl ion
([Fe
![]() O]
O]![]() ) as the oxidative
intermediate.[157]
The reactions are of industrial interest for
their applications in waste water treatment and paper bleaching,
but also in biological processes are their oxygen activation
abilities recognized. Recently, much attention has been given to
iron containing biological molecules that efficiently catalyze the
oxidation of organic substrates, such as the antitumor drug
bleomycin[158],
cytochrome oxydase[159,160,161,162]
and methane monooxygenases[163,164]. Biomimetically designed ligand environments of
iron complexes[132,131] are studied to optimize industrial catalysts,
but also other chelated[165,166] and
un-chelated[167,136,139]
iron(II)/H
) as the oxidative
intermediate.[157]
The reactions are of industrial interest for
their applications in waste water treatment and paper bleaching,
but also in biological processes are their oxygen activation
abilities recognized. Recently, much attention has been given to
iron containing biological molecules that efficiently catalyze the
oxidation of organic substrates, such as the antitumor drug
bleomycin[158],
cytochrome oxydase[159,160,161,162]
and methane monooxygenases[163,164]. Biomimetically designed ligand environments of
iron complexes[132,131] are studied to optimize industrial catalysts,
but also other chelated[165,166] and
un-chelated[167,136,139]
iron(II)/H![]() O
O![]() complex in aqueous solution are studied
as well as oxidations by bare iron-oxo species
in the gas phase[168,169,170] in order
to reveal the reaction mechanisms.
Despite the numerous studies over more than 60 years, the controversy
remains as there have not yet been definitive experiments to
distinguish between the proposed alternatives. The main experimental
difficulty, the extremely short life times of the reaction
intermediates, is not a problem for computer simulation methods.
A second difficulty is the sensitivity of Fenton chemistry to the
reaction conditions, such as the pH, the metal ligands (chelating
agents), and the nature of organic substrates,
which complicate the development of a microscopic model.
We have therefore first restricted ourselves in a previous study to
the mechanism of the basic reaction between hydrated Fe(II) and hydrogen
peroxide in vacuo[145].
Subsequently, the influence of other parameters on the mechanism
will be the subject of study, starting with the solvent effect
of aqueous solution in the present study. A preliminary account has
appeared in ref. bernd3.
complex in aqueous solution are studied
as well as oxidations by bare iron-oxo species
in the gas phase[168,169,170] in order
to reveal the reaction mechanisms.
Despite the numerous studies over more than 60 years, the controversy
remains as there have not yet been definitive experiments to
distinguish between the proposed alternatives. The main experimental
difficulty, the extremely short life times of the reaction
intermediates, is not a problem for computer simulation methods.
A second difficulty is the sensitivity of Fenton chemistry to the
reaction conditions, such as the pH, the metal ligands (chelating
agents), and the nature of organic substrates,
which complicate the development of a microscopic model.
We have therefore first restricted ourselves in a previous study to
the mechanism of the basic reaction between hydrated Fe(II) and hydrogen
peroxide in vacuo[145].
Subsequently, the influence of other parameters on the mechanism
will be the subject of study, starting with the solvent effect
of aqueous solution in the present study. A preliminary account has
appeared in ref. bernd3.
In the gas-phase study, we have analyzed the reaction of H![]() O
O![]() with Fe
with Fe![]() from the point of view of the energetics and the
electronic and geometric structure using Density Functional Theory
(DFT) calculations. As the primary step in the proposed models usually is
the coordination of the hydrogen peroxide to iron,
from the point of view of the energetics and the
electronic and geometric structure using Density Functional Theory
(DFT) calculations. As the primary step in the proposed models usually is
the coordination of the hydrogen peroxide to iron,
we started from a configuration of H![]() O
O![]() coordinated to
a pentaaqua iron(II) complex. It turned out that the complexed
hydrogen peroxide easily dissociates but the formation of free OH.
radicals is from an energetic point of view very unlikely.
Instead, the formation of the ferryl ion
([(H
coordinated to
a pentaaqua iron(II) complex. It turned out that the complexed
hydrogen peroxide easily dissociates but the formation of free OH.
radicals is from an energetic point of view very unlikely.
Instead, the formation of the ferryl ion
([(H![]() O)
O)![]() Fe
Fe
![]() =O]
=O]![]() ) plus water is exothermic by
) plus water is exothermic by
![]() = 28 kcal/mol.[145]
= 28 kcal/mol.[145]
In practice however,
these reactions take place in aqueous solution and the solvent effects
are expected to be an important factor in the balance between the
competing reactions. Already in our gas phase study we have observed that
adding a single water molecule could facilitate the formation of the
ferryl ion from the Fenton reagent by lowering the reaction barrier of the proton
transfer process.
In the present study, we introduce the effects of a complete water solution
environment and report the oxidizing intermediates formed in the
reaction of Fe![]() and hydrogen peroxide in water at
and hydrogen peroxide in water at ![]() =300K predicted by
computer simulation. To be able to model correctly the active role of
the solvent water molecules in the Fenton reaction, the method of choice
for this study is the Car-Parrinello (CP) method.[49] The CP method
applies classical molecular dynamics (MD), computing the inter- and
intramolecular forces from the electronic structure determined quantum mechanically
with Density Functional Theory (DFT) in an efficient way.
The CP method is therefore regarded as an ab initio (DFT) molecular
dynamics (AIMD) method. This method has proven to be
a very valuable tool to study at a microscopic scale structure and dynamics of
water[50,51], (metal-) ions in water[117,52,53,172]
and simple chemical reactions in aqueous solution.[144,54,55]
The full strength of AIMD becomes apparent in the simulation of bond breaking and
making as e.g. a proton or OH. radical jumps through an aqueous solution;
something that is practically impossible to model with classical force fields.
=300K predicted by
computer simulation. To be able to model correctly the active role of
the solvent water molecules in the Fenton reaction, the method of choice
for this study is the Car-Parrinello (CP) method.[49] The CP method
applies classical molecular dynamics (MD), computing the inter- and
intramolecular forces from the electronic structure determined quantum mechanically
with Density Functional Theory (DFT) in an efficient way.
The CP method is therefore regarded as an ab initio (DFT) molecular
dynamics (AIMD) method. This method has proven to be
a very valuable tool to study at a microscopic scale structure and dynamics of
water[50,51], (metal-) ions in water[117,52,53,172]
and simple chemical reactions in aqueous solution.[144,54,55]
The full strength of AIMD becomes apparent in the simulation of bond breaking and
making as e.g. a proton or OH. radical jumps through an aqueous solution;
something that is practically impossible to model with classical force fields.
This paper is structured as follows: in the next section (section 5.2),
we start with the computational details. Then, in order to asses the accuracy
of the Car-Parrinello method in the description of the solvent structure
around an iron complex, we compare structural properties of FeCl![]() in water with
experimental data (section 5.2.1).
The presentation of our results (section 5.3) begins with static
DFT calculations of the energetics for the elementary reactions to
produce the OH. radical and the ferryl ion with the Fenton reagent in vacuo,
in section 5.3.1.
Our main results, the reaction mechanisms of iron(II) and H
in water with
experimental data (section 5.2.1).
The presentation of our results (section 5.3) begins with static
DFT calculations of the energetics for the elementary reactions to
produce the OH. radical and the ferryl ion with the Fenton reagent in vacuo,
in section 5.3.1.
Our main results, the reaction mechanisms of iron(II) and H![]() O
O![]() in
aqueous solution at room temperature, are presented in sections 5.3.2,
5.3.3 and 5.3.4. We first show the
spontaneous formation of iron(IV)oxo species in an AIMD simulation when we start
from H
in
aqueous solution at room temperature, are presented in sections 5.3.2,
5.3.3 and 5.3.4. We first show the
spontaneous formation of iron(IV)oxo species in an AIMD simulation when we start
from H![]() O
O![]() coordinated to pentaaqua iron(II), via an iron(IV) dihydroxo
intermediate, with and without solvent molecules playing an active role in
the mechanism (sections 5.3.2 and 5.3.3, respectively).
In section 5.3.4, we also include the coordination process,
by starting from separated reactants in water.
A summary and conclusions are given in section 5.4.
coordinated to pentaaqua iron(II), via an iron(IV) dihydroxo
intermediate, with and without solvent molecules playing an active role in
the mechanism (sections 5.3.2 and 5.3.3, respectively).
In section 5.3.4, we also include the coordination process,
by starting from separated reactants in water.
A summary and conclusions are given in section 5.4.