The energetics of the elementary reactions in vacuo, were calculated, using the Slater type orbital based ADF package.[70] The same exchange-correlation functional was used for the static DFT calculations as in the ab initio (DFT) molecular dynamics calculations.[67,38] The Kohn-Sham orbitals were expanded in a large even-tempered all-electron Slater-type basis set containing: 4 s, 2 p, and 1 d functions for hydrogen; 6 s, 4 p, 2 d, and 1 f functions for oxygen; and 11 s, 7 p, 5 d, and 1 f functions for iron[175]. The results are compiled in table 5.2. Some of these data are already given in ref. franco1; small differences between table 5.2 and ref. franco1 are due to the better basis set used for the present results in the table.
We see that it costs 60 kcal/mol to dissociate hydrogen peroxide into two
hydroxyl radicals in the gas phase. The inclusion of the zero-point energy
correction gives 54 kcal/mol, in reasonable agreement with the experimental
value at 25![]() C of 51.2 kcal/mol.[142]
The iron catalyzed production of
a hydroxyl radical and a hydroxo ligand starting from a pentaaqua
iron(II) hydrogen peroxide complex costs 21 kcal/mol.
The reduction by 39 kcal/mol is obtained from the
much stronger Fe
C of 51.2 kcal/mol.[142]
The iron catalyzed production of
a hydroxyl radical and a hydroxo ligand starting from a pentaaqua
iron(II) hydrogen peroxide complex costs 21 kcal/mol.
The reduction by 39 kcal/mol is obtained from the
much stronger Fe
![]() -OH
-OH![]() bond compared
to the Fe
bond compared
to the Fe
![]() -H
-H![]() O
O![]() bond: E
bond: E![]() A+C-D in the table.
Still, free OH. radical formation in the gas phase remains very unfavorable.
However, the remaining 21 kcal/mol can be overcome by formation of a second
Fe-OH
A+C-D in the table.
Still, free OH. radical formation in the gas phase remains very unfavorable.
However, the remaining 21 kcal/mol can be overcome by formation of a second
Fe-OH![]() bond if the oxygen-oxygen cleavage is accompanied by hydrogen
abstraction from an adjacent water ligand
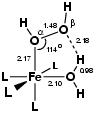
(see also figure 5.2) by the
bond if the oxygen-oxygen cleavage is accompanied by hydrogen
abstraction from an adjacent water ligand
(see also figure 5.2) by the ![]() -oxygen to form
dihydroxo tetraaqua iron(IV) and water. This step is exothermic
by 29 kcal/mol with a small reaction barrier of 6 kcal/mol
(reaction F in the table).
The pentaaqua iron(IV) oxo complex (ferryl ion) is then easily
produced from the dihydroxo complex in a second exothermic step,
via an internal proton transfer reaction
(reaction H in the table). The water molecule produced in the first
step is strongly bonded to the complex via the hydrogen of an OH
-oxygen to form
dihydroxo tetraaqua iron(IV) and water. This step is exothermic
by 29 kcal/mol with a small reaction barrier of 6 kcal/mol
(reaction F in the table).
The pentaaqua iron(IV) oxo complex (ferryl ion) is then easily
produced from the dihydroxo complex in a second exothermic step,
via an internal proton transfer reaction
(reaction H in the table). The water molecule produced in the first
step is strongly bonded to the complex via the hydrogen of an OH![]() ligand.
By abstracting the hydrogen of this OH
ligand.
By abstracting the hydrogen of this OH![]() ligand and passing on another
hydrogen to the second OH
ligand and passing on another
hydrogen to the second OH![]() ligand, this water molecule facilitates
step two and keeps the barrier for this transformation as low as 3.5 kcal/mol.
Without the water molecule, the barrier for the second step is 18 kcal/mol.
The total reaction energy for the formation of the ferryl ion from
the pentaaqua iron(II)hydrogen peroxide is -34 kcal/mol (F + H in the table).
ligand, this water molecule facilitates
step two and keeps the barrier for this transformation as low as 3.5 kcal/mol.
Without the water molecule, the barrier for the second step is 18 kcal/mol.
The total reaction energy for the formation of the ferryl ion from
the pentaaqua iron(II)hydrogen peroxide is -34 kcal/mol (F + H in the table).
|
|
||||
| Gas phase reaction | ||||
|
|
||||
|
|
||||
| A | H |
2 OH. | 59.9 | |
|
|
||||
| B | [(H |
22.1 | ||
|
|
||||
| C | [(H |
22.8 | ||
|
|
||||
| D | [(H |
61.9 | ||
|
|
||||
| E | [(H |
20.7 | ||
|
|
||||
| F | [(H |
-29.3 | ||
|
|
||||
| G | [(H |
-1.3 | ||
|
|
||||
| H | [(H |
-4.5 | ||
|
|
||||
| I | [(H |
-6.7 | ||
|
These energies show that the ferryl ion is the more likely candidate for the
oxidating species in Fenton chemistry instead of the OH. radical.
The gas phase study[145] has also strongly indicated that solvent effects
are important for the energetics, a striking example being the lowering of
the barrier of the second step by inclusion of one water molecule
in a ``second solvation shell'' position. Not only the reaction barriers
will be modified by solvent effects but also the overall energetics.
For instance, a large part of the 34 kcal/mol exothermicity of
the ferryl ion formation in the gas phase originates from the very
strongly bound ``second solvation shell'' water molecule produced
in the first step (reaction F). This interaction energy is in the order
of 28 kcal/mol (compare e.g. reaction F and G), which is much
larger than for a typical hydrogen bond (this has been elucidated in
ref. franco1). In aqueous solution, this second solvation shell position
would already be taken by a solvent molecule, so that the overall reaction
energetics for the ferryl ion formation in solution is more likely to be
in the order of -8 kcal/mol (G + I in the table).
Of course, we also have to keep in mind that the solvation of the reactant
(pentaaqua iron(II) hydrogen peroxide)
cannot be expected to be the same as the solvation of the products (either
[(H![]() O)
O)![]() Fe
Fe
![]() O)]
O)]![]() and H
and H![]() O
or [(H
O
or [(H![]() O)
O)![]() Fe
Fe
![]() OH)]
OH)]![]() and OH.), so that the
reaction energies for reactions E, G and I will be modified in
aqueous solution. Solvent effects can therefore make the preference
for the ferryl ion formation over the free OH. radical mechanism
less prominent, or even make the two mechanisms competitive, in contrast
to the gas phase Fenton chemistry. Whether this is the case, is the
topic of the present study.
and OH.), so that the
reaction energies for reactions E, G and I will be modified in
aqueous solution. Solvent effects can therefore make the preference
for the ferryl ion formation over the free OH. radical mechanism
less prominent, or even make the two mechanisms competitive, in contrast
to the gas phase Fenton chemistry. Whether this is the case, is the
topic of the present study.