


Next: Techniques
Up: Introduction
Previous: Our model
Contents
This thesis is about the study of chemical reactions in water
without getting our hands wet. Solvent effects can have a very
large influence on the thermodynamics and the kinetics of chemical
reactions in water, but are usually poorly described or even
neglected completely in theoretical models. Moreover, the understanding
of solvation shell dynamics and solvent effects on chemistry on a
microscopic level is in general still rather poor. Having said that,
we will continue in the next chapter with the introduction
of the techniques applied for our study. In particular, A) the
Car-Parrinello technique which efficiently solves the problem of
following the motions of the nuclei while simultaneously updating
the electronic structure allowing for the simulation of chemical
reactions in the condensed
phase and B) the statistical thermodynamics techniques to solve the
``rare event'' problem of observing the crossing of a chemical
reaction barrier whose height is several order of kT with the
Car-Parrinello molecular dynamics technique. In chapter 3,
we start with the investigation of the solvent effects on a textbook
S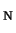 2 reaction. Chemically, this type of reaction is
very well understood: the solvent effects are very large, leading
to a decrease of the reaction rate of 13 orders of magnitude (!)
in aqueous solution with respect to this reaction in the gas
phase. We show that we can accurately calculate the solvent-solute
interactions and compute the solvent effects on the reaction free
energy barrier in aqueous solution. Moreover, we rationalize the
strong solvent effects from the structural changes in the solvent
during the reaction. After this prototype, yet very instructive,
S
2 reaction. Chemically, this type of reaction is
very well understood: the solvent effects are very large, leading
to a decrease of the reaction rate of 13 orders of magnitude (!)
in aqueous solution with respect to this reaction in the gas
phase. We show that we can accurately calculate the solvent-solute
interactions and compute the solvent effects on the reaction free
energy barrier in aqueous solution. Moreover, we rationalize the
strong solvent effects from the structural changes in the solvent
during the reaction. After this prototype, yet very instructive,
S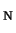 2 reaction, we move on to the study of transition
metal catalyzed oxidation reactions in aqueous solution, a short
introduction of which is given in chapter 4.
In particular, we investigate the solvent effects on the catalyzed
activation of hydrogen peroxide by iron(II) in chapter 5
and by iron(III) in chapter 6, also known as
Fenton and Fenton-like chemistry, respectively. We show that in these
cases the solvent molecules are actively involved in the reaction
mechanisms, catalyzing the chemical transformations on the metal
complexes via hydrogen and proton abstraction reactions. Spontaneous
hydrolysis of the acidic metal complexes is seen to play an
important role, again a process which cannot be observed without
an accurate incorporation of the aqueous environment. We elucidate
the surprisingly different reaction mechanisms for Fenton and Fenton-like
chemistry in water and show that in the Fe
2 reaction, we move on to the study of transition
metal catalyzed oxidation reactions in aqueous solution, a short
introduction of which is given in chapter 4.
In particular, we investigate the solvent effects on the catalyzed
activation of hydrogen peroxide by iron(II) in chapter 5
and by iron(III) in chapter 6, also known as
Fenton and Fenton-like chemistry, respectively. We show that in these
cases the solvent molecules are actively involved in the reaction
mechanisms, catalyzing the chemical transformations on the metal
complexes via hydrogen and proton abstraction reactions. Spontaneous
hydrolysis of the acidic metal complexes is seen to play an
important role, again a process which cannot be observed without
an accurate incorporation of the aqueous environment. We elucidate
the surprisingly different reaction mechanisms for Fenton and Fenton-like
chemistry in water and show that in the Fe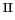 /H
/H O
O case
the much contested [Fe
case
the much contested [Fe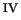 O]
O] ion is the predominant
oxidative intermediate and not the hydroxyl radical as is generally
believed. Instead, in the Fe
ion is the predominant
oxidative intermediate and not the hydroxyl radical as is generally
believed. Instead, in the Fe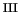 /H
/H O
O case, an H
case, an H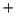 is donated to the solvent to produce the iron(III)hydroperoxo species as the
primary intermediate. In these chapters (chapter 5
and 6), we present a number of illuminating
and illustrative simulations of the dynamics of the Fenton and the
Fenton-like reactions in aqueous solution at room temperature.
However, concern is in place with the representativeness of such
reactive trajectories, in view of the unphysical geometric constraints
applied in the construction of the systems in order to overcome the
earlier mentioned rare event problem. Therefore, chapter 7
is devoted to the generation of representative reactive trajectories with
no memory of the artificial construction of the system by way of the new
transition path sampling technique. In chapter 8,
we study the oxidation of methane to methanol by the ferryl ion
([Fe
is donated to the solvent to produce the iron(III)hydroperoxo species as the
primary intermediate. In these chapters (chapter 5
and 6), we present a number of illuminating
and illustrative simulations of the dynamics of the Fenton and the
Fenton-like reactions in aqueous solution at room temperature.
However, concern is in place with the representativeness of such
reactive trajectories, in view of the unphysical geometric constraints
applied in the construction of the systems in order to overcome the
earlier mentioned rare event problem. Therefore, chapter 7
is devoted to the generation of representative reactive trajectories with
no memory of the artificial construction of the system by way of the new
transition path sampling technique. In chapter 8,
we study the oxidation of methane to methanol by the ferryl ion
([Fe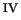 O]
O] ), i.e. the active intermediate proposed
for the Fenton reaction in chapter 5 and find that again
the water environment has a large effect on the chemistry via the
hydrophobic interactions with the hydrocarbon species and the
solvated metal oxo and hydroxo complexes. Also, we confirm the mechanistic
similarities between the organic oxidations by Fenton's reagent and those
by such enzymes as MMO (methane mono-oxygenase) and cytochrome P450,
as was recently suggested in literature.
This thesis ends with a summary (also in Dutch) and
acknowledgments to the people who have contributed to this work.
), i.e. the active intermediate proposed
for the Fenton reaction in chapter 5 and find that again
the water environment has a large effect on the chemistry via the
hydrophobic interactions with the hydrocarbon species and the
solvated metal oxo and hydroxo complexes. Also, we confirm the mechanistic
similarities between the organic oxidations by Fenton's reagent and those
by such enzymes as MMO (methane mono-oxygenase) and cytochrome P450,
as was recently suggested in literature.
This thesis ends with a summary (also in Dutch) and
acknowledgments to the people who have contributed to this work.



Next: Techniques
Up: Introduction
Previous: Our model
Contents
Bernd Ensing
2003-06-13