Previously, we have shown that the ferryl ion ([Fe
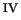
O]

) is easily
produced from Fenton's reagent (
i.e. a mixture of Fe

ions and
H

O

in aqueous solution), using DFT and Car-Parrinello MD
calculations. In order to definitely conclude that
the ferryl ion is indeed the active species in oxidation reactions with Fenton's
reagent, we have in the present study studied the reactivity of the ferryl
ion towards organic substrates, in particular the oxidation of methane to
methanol. Our static DFT calculations on the
[(H

O)
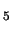
Fe
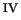
O]

-CH
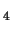
complex
in vacuo show a strong
prevalance for
the oxygen-rebound mechanism over the methane-coordination mechanism, which is
in agreement with the results for methane oxidation by bio-catalysts MMO and P450,
but not with those for methane oxidation by bare metal-oxo ions. The highest energy
barrier in the oxygen-rebound mechanism is only 3 kcal/mol, whereas in the methane
coordination mechanism the highest barrier is 23 kcal/mol. Overall the oxidation reaction
energy is downhill by 47 kcal/mol. We also have computed the free energy barrier of the
H-abstraction reaction from methane by the ferryl ion (
i.e. the first step
in the rebound mechanism) in aqueous solution by the method of constrained
(first principles) molecular dynamics. The free energy barrier of 22 kcal/mol in
solution is significantly higher that it is
in vacuo. Nevertheless, in
combination with our previous work, we must conclude that the ferryl ion is indeed
the active intermediate in Fenton chemistry.