The formation of active intermediates from the Fenton-like reagent (a mixture
of iron(III) ions and hydrogen peroxide) in aqueous solution has been investigated
using static DFT calculations and Car-Parrinello molecular dynamics simulations.
We show the spontaneous formation of the iron(III)hydroperoxo intermediate in
a first step. The Fenton-like reaction thus proceeds very differently compared to
Fenton's reagent (
i.e. the Fe
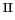
/H

O

mixture), for which
we have recently shown that the first step is the spontaneous O-O lysis of
hydrogen peroxide when coordinated to iron(II) in water. For the second step in the
reaction mechanism of the Fenton-like reagent, we compare the possibilities of
homolysis and heterolysis of the O-O bond and the Fe-O bond of the produced
[(H

O)
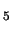
Fe
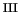
OOH]

intermediate. We find that
concomitant hydrolysis of the reacting species plays a crucial role and,
taking this into account, that O-O homolysis
([(H

O)
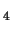
(OH)Fe
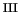
OOH]
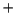
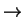
[(H

O)
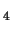
(OH)Fe
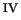
O]
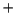
+ OH.)
in vacuo is most
favorable with
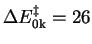
kcal/mol. However, also
the proper inclusion of the solvent effects is important.
We have therefore calculated the free energy barrier for the O-O homolysis of the
iron(III)hydroperoxo intermediate in aqueous solution at
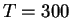
K, using the
method of constrained molecular dynamics and thermodynamic integration,
resulting in
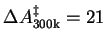
kcal/mol. Analysis of the vibrational
spectra of the high-spin (
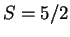
) and the low-spin (
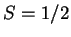
) Fe(III)OOH
intermediate confirms the in the literature suggested effect of the spin-state
on the Fe-O and O-O bond strengths. In fact, we predict that with ligands
inducing a low-spin iron(III)hydroperoxo intermediate, the barrier for the
O-O homolysis will be even significantly lower.