


Next: The first reaction step
Up: Results
Previous: Results
Contents
Elementary Fenton and Fenton-like reactions in vacuo
We have computed the reaction energies of the elementary Fenton and Fenton-like
reactions of the hydrated iron complexes in vacuo, among which the ones
mentioned in the introduction (equations 6.1-6.6),
and compiled the results in table 6.1. The first two columns
of numbers on the left-hand-side show the reaction energies in kcal/mol with
an iron(II) complex and an iron(III) complex as the reactant, respectively.
Hydrated metal ions can be acidic, the acidity increasing with increasing
oxidation state of the metal ion. For example, the acidity constant of
[Fe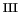 (H
(H O)
O)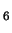 ]
]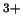 equals p
equals p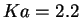 [154], so that in
aqueous solution hydrolysis easily takes place to form the iron(III)hydroxo complex
[Fe
[154], so that in
aqueous solution hydrolysis easily takes place to form the iron(III)hydroxo complex
[Fe (H
(H O)
O) (OH)]
(OH)] and a free hydronium ion.
As we shall see, the lowering of the total charge
on the metal complex from 3+ to 2+ has a significant effect on the reactivity.
The reaction energies of the present elementary reactions starting from such an
iron(III)hydroxo complex are given in the last column of
table 6.1. Deprotonation of an iron(II) complex is less likely.
The acidity constant of hexaaquairon(II) is
p
and a free hydronium ion.
As we shall see, the lowering of the total charge
on the metal complex from 3+ to 2+ has a significant effect on the reactivity.
The reaction energies of the present elementary reactions starting from such an
iron(III)hydroxo complex are given in the last column of
table 6.1. Deprotonation of an iron(II) complex is less likely.
The acidity constant of hexaaquairon(II) is
p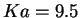 [143], so that the [Fe
[143], so that the [Fe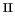 (H
(H O)
O)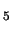 (OH)]
(OH)]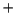 complex forms an improbable starting species.
complex forms an improbable starting species.
Starting from hexaaquairon complexes, we see after comparing reactions A and B,
that the ligand exchange of a water ligand by hydrogen peroxide is almost
thermoneutral, but that the water (and H O
O ) ligands are much stronger
bonded to the 3+ charged iron(III) complexes than to the 2+ charged iron(II)
and iron(III)hydroxo complexes (by more than 20 kcal/mol). The production of a
free hydroxyl radical, starting from hydrogen peroxide coordinated to iron(II)
(as in the Haber and Weiss mechanism, reaction 6.1) costs
20.7 kcal/mol (reaction C in the table), a reduction of 39.2 kcal/mol with
respect to the dissociation of free hydrogen peroxide into two
hydroxyl radicals (
) ligands are much stronger
bonded to the 3+ charged iron(III) complexes than to the 2+ charged iron(II)
and iron(III)hydroxo complexes (by more than 20 kcal/mol). The production of a
free hydroxyl radical, starting from hydrogen peroxide coordinated to iron(II)
(as in the Haber and Weiss mechanism, reaction 6.1) costs
20.7 kcal/mol (reaction C in the table), a reduction of 39.2 kcal/mol with
respect to the dissociation of free hydrogen peroxide into two
hydroxyl radicals (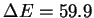 kcal/mol at the same level of theory and
54 kcal/mol including the zero point energy, in reasonable agreement with the
experimental value at 25
kcal/mol at the same level of theory and
54 kcal/mol including the zero point energy, in reasonable agreement with the
experimental value at 25 C of 51.2 kcal/mol). For hydrogen peroxide
coordinated to pentaaquairon(III) on the other hand, does the O-O dissociation
and free OH. radical formation not lead to a reduction compared to free
H
C of 51.2 kcal/mol). For hydrogen peroxide
coordinated to pentaaquairon(III) on the other hand, does the O-O dissociation
and free OH. radical formation not lead to a reduction compared to free
H O
O dissociation, but is even slightly more endothermic (by 1 kcal/mol).
The OH. radical produced in reaction C can also abstract the hydrogen from
the produced hydroxo ligand to form the ferryl ion
([Fe
dissociation, but is even slightly more endothermic (by 1 kcal/mol).
The OH. radical produced in reaction C can also abstract the hydrogen from
the produced hydroxo ligand to form the ferryl ion
([Fe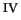 (H
(H O)
O)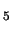 O]
O] ) and a water molecule, following the Bray
and Gorin mechanism (reaction 6.2) when starting from
[Fe
) and a water molecule, following the Bray
and Gorin mechanism (reaction 6.2) when starting from
[Fe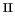 (H
(H O)
O)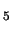 (H
(H O
O )]
)] or the
[Fe
or the
[Fe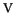 (H
(H O)
O)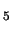 O]
O]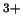 species and H
species and H O when starting from
[Fe
O when starting from
[Fe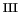 (H
(H O)
O)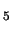 (H
(H O
O )]
)]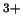 (reaction D in the table).
In the first case, the overall reaction is exothermic by 8 kcal/mol, but starting
with iron(III), the formation of the oxo species is again energetically very
unfavorable (
(reaction D in the table).
In the first case, the overall reaction is exothermic by 8 kcal/mol, but starting
with iron(III), the formation of the oxo species is again energetically very
unfavorable (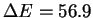 kcal/mol). These numbers clearly show, in the first
place, that the highly reactive OH. radical and high-valent iron oxo species
are much more easily formed from Fenton's reagent (Fe
kcal/mol). These numbers clearly show, in the first
place, that the highly reactive OH. radical and high-valent iron oxo species
are much more easily formed from Fenton's reagent (Fe /H
/H O
O ) than from
the Fenton-like reagent (Fe
) than from
the Fenton-like reagent (Fe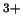 /H
/H O
O ), confirming the experimentally
observed difference in oxidative reactivity between the two reagents. In the
second place, reactions C and D indicate that the ferryl ion is a much more
likely candidate for the active species in Fenton chemistry than the free
OH. radical. In refs franco1,bernd3,bernd4,bernd5, we have discussed the
Fenton reagent more extensively, and we have shown that in the two-step process that
leads to formation of the ferryl ion, the highest of the two transition states
is only 6 kcal/mol. We have also
investigated the reactivity of the ferryl ion towards organic substrates by
simulating the oxidation of methane to methanol by the ferryl ion[198].
We will now continue to focus solely on the Fenton-like reagent.
), confirming the experimentally
observed difference in oxidative reactivity between the two reagents. In the
second place, reactions C and D indicate that the ferryl ion is a much more
likely candidate for the active species in Fenton chemistry than the free
OH. radical. In refs franco1,bernd3,bernd4,bernd5, we have discussed the
Fenton reagent more extensively, and we have shown that in the two-step process that
leads to formation of the ferryl ion, the highest of the two transition states
is only 6 kcal/mol. We have also
investigated the reactivity of the ferryl ion towards organic substrates by
simulating the oxidation of methane to methanol by the ferryl ion[198].
We will now continue to focus solely on the Fenton-like reagent.
The formation of the iron(III)hydroperoxo species from iron(III)hydrogen-peroxide
in aqueous solution (reaction 6.3), which is believed to
be the initial step in Fenton-like chemistry
(reaction E in table 6.1), is poorly modeled in vacuo. The
absolute reaction energies of such charge separation reactions are typically highly
overestimated, due to the omission of the screening of the solvent and the
energies of solvation. The hydrolysis of hexaaquairon(III) for instance,
forming pentaaqua hydroxo iron(III) by donating a proton to a water molecule
in vacuo, results in an energy gain of 145 kcal/mol, whereas the experimental
acidity constant of p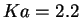 indicates an (free) energy loss of 3 kcal/mol.
However, we can nevertheless compare the reaction energies of charge separation
processes for which the solvent effects are expected to be similar. Hydrolysis
of coordinated H
indicates an (free) energy loss of 3 kcal/mol.
However, we can nevertheless compare the reaction energies of charge separation
processes for which the solvent effects are expected to be similar. Hydrolysis
of coordinated H O in pentaaquairon(III)hydrogen-peroxide (reaction F in
table 6.1), for example, is not expected to be much different
from the hydrolysis of hexaaquairon(III), and the reaction energies in vacuo are
indeed in both cases found to be -145 kcal/mol.
Now, we can compare reaction E and F, assuming
no large differences in energies of solvation for the products, and conclude that
the formation of the iron(III)hydroperoxo species in aqueous solution indeed is a
likely initial step in Fenton-like chemistry, and that the hydrolysis of the
H
O in pentaaquairon(III)hydrogen-peroxide (reaction F in
table 6.1), for example, is not expected to be much different
from the hydrolysis of hexaaquairon(III), and the reaction energies in vacuo are
indeed in both cases found to be -145 kcal/mol.
Now, we can compare reaction E and F, assuming
no large differences in energies of solvation for the products, and conclude that
the formation of the iron(III)hydroperoxo species in aqueous solution indeed is a
likely initial step in Fenton-like chemistry, and that the hydrolysis of the
H O
O ligand is probably even favored over the hydrolysis of a H
ligand is probably even favored over the hydrolysis of a H O ligand.
Nevertheless, we want to stress that the proper inclusion of the solvent effects
is required to accurately model this first step in Fenton-like chemistry.
O ligand.
Nevertheless, we want to stress that the proper inclusion of the solvent effects
is required to accurately model this first step in Fenton-like chemistry.
Reactions G till J in the table are possible reactions of a second step, in which
the iron(III)hydroperoxo species forms very reactive particles such as radicals
and high-valent iron oxo species. We see that the unscreened charge separation processes
of Fe-O bond or O-O bond heterolysis (reactions H and J, respectively) again results in
very high values for the reaction energies in vacuo (this time uphill),
which makes it impossible to compare these reactions with the homolysis equivalents
(reactions G and I, respectively), although we doubt that inclusion of the solvent screening
and solvation energies will bring the reaction energies of reaction H and J in aqueous solution
below 50 kcal/mol. The energies for the homolysis of the Fe-O or O-O bond of
36.8 kcal/mol and 42.6 kcal/mol are also rather high. An important difference between
the two reactions is that, although they both produce highly reactive radicals,
in the Fe-O homolysis (G) the formal oxidation state of iron is lowered, whereas
in the O-O homolysis (I) the formal oxidation state of iron increases. As the
acidity of hydrated metal ions increases with the oxidation state of the metal
(see before), hydrolysis of the metal complex works in opposite directions for
the two homolysis reactions. Taking the hydrolysis effect into account results in
O-O homolysis forming the most probable second step in Fenton-like chemistry, with
a reaction energy of 26.1 kcal/mol in vacuo, comparable to the initial ligand
expulsion step, reaction A (see the third column of table 6.1 for the
reaction energies when first hydrolysis of an H O ligand has occurred).
As in this reaction both OH. radicals and ferryl ions are
formed, it is particularly interesting to study this O-O homolysis in more detail
with inclusion of the water environment. In section 6.3.3, we will
compute the free energy barrier for the O-O homolysis reaction in aqueous solution,
and will then consider the role of (simultaneous) hydrolysis of a H
O ligand has occurred).
As in this reaction both OH. radicals and ferryl ions are
formed, it is particularly interesting to study this O-O homolysis in more detail
with inclusion of the water environment. In section 6.3.3, we will
compute the free energy barrier for the O-O homolysis reaction in aqueous solution,
and will then consider the role of (simultaneous) hydrolysis of a H O ligand
in more detail.
O ligand
in more detail.
Summarizing, the static DFT calculations show that for iron(III)hydrogen-peroxide
the direct formation of the OH. radical (C) or the high-valent iron oxo species(D)
is energetically much less favorable than for iron(II)hydrogen-peroxide. Secondly,
the acidity of iron(III) complexes is expected to play an important role as the
hydrolysis of a water ligand lowers the reaction energies dramatically, particularly
in the case of the iron(V)oxo complex formation (D). In the third place, hydrolysis
of the H O
O ligand of [Fe
ligand of [Fe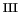 (H
(H O)
O)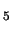 (H
(H O
O )]
)]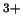 ,
producing the iron(III)hydroperoxo species (E) is energetically favored over
hydrolysis of a water ligand (F). In the next section, we will show that in aqueous
solution indeed the hydrolysis of the H
,
producing the iron(III)hydroperoxo species (E) is energetically favored over
hydrolysis of a water ligand (F). In the next section, we will show that in aqueous
solution indeed the hydrolysis of the H O
O ligand (E) forms the initial step
in the Fenton-like chemistry, so that for the next step the transformation of the
iron(III)hydroperoxo species becomes important. Fourth, possible second-step transformations
are the homolysis of the Fe-O bond (G) and the O-O bond (I) of which the latter becomes
particularly interesting when a second hydrolysis (of a water ligand) takes place.
We will investigate the O-O bond homolysis and the simultaneous second hydrolysis
in aqueous solution in section 6.3.3.
ligand (E) forms the initial step
in the Fenton-like chemistry, so that for the next step the transformation of the
iron(III)hydroperoxo species becomes important. Fourth, possible second-step transformations
are the homolysis of the Fe-O bond (G) and the O-O bond (I) of which the latter becomes
particularly interesting when a second hydrolysis (of a water ligand) takes place.
We will investigate the O-O bond homolysis and the simultaneous second hydrolysis
in aqueous solution in section 6.3.3.



Next: The first reaction step
Up: Results
Previous: Results
Contents
Bernd Ensing
2003-06-13