We assume, as is usually done, that the initial step in Fenton and
Fenton-like chemistry is the nucleophilic addition of hydrogen
peroxide to the iron complex by exchanging with a water ligand in the
hydration shell[196]. In the next section (6.3.1),
we discuss the energetics of various reactions starting from the
[Fe![]() (H
(H![]() O)
O)![]() (H
(H![]() O
O![]() )]
)]![]() and the
[Fe
and the
[Fe![]() (H
(H![]() O)
O)![]() (H
(H![]() O
O![]() )]
)]![]() complexes in order to
investigate the thermodynamic possibilities and impossibilities.
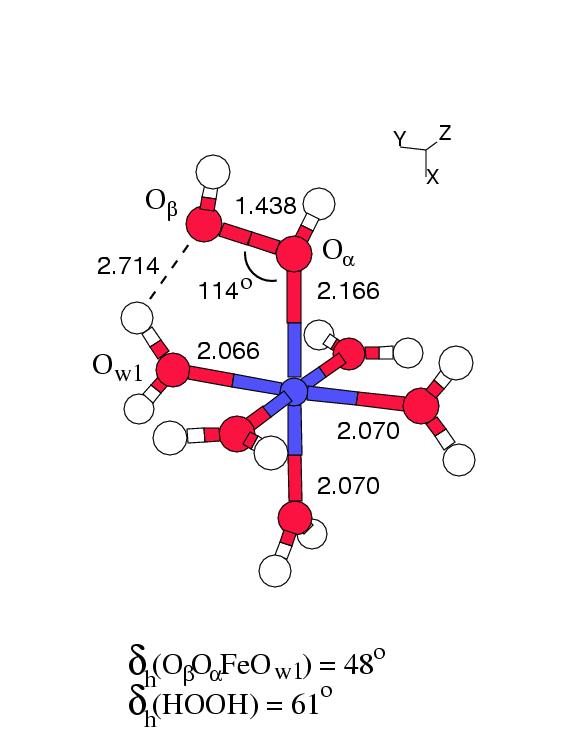
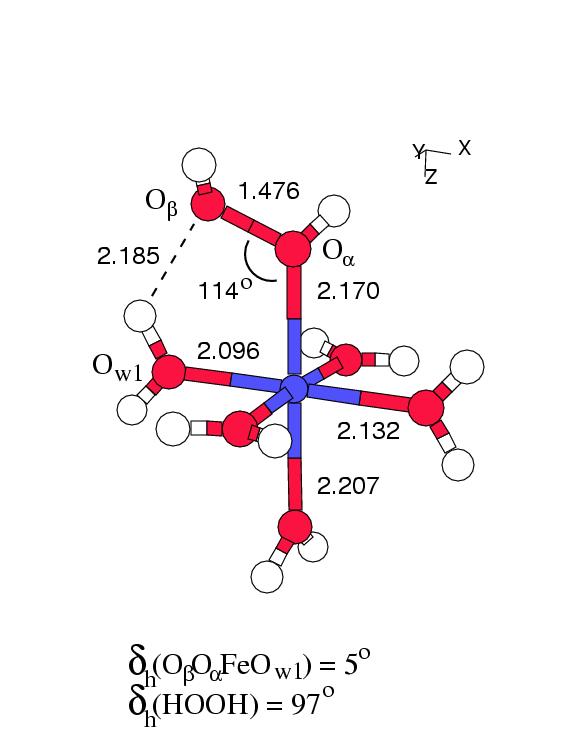
Figure 6.1 shows the geometry
optimized structures of these two complexes in vacuo.
Later on, in section 6.3.2, we will show
an illustrative pathway of the reaction of H
complexes in order to
investigate the thermodynamic possibilities and impossibilities.
Figure 6.1 shows the geometry
optimized structures of these two complexes in vacuo.
Later on, in section 6.3.2, we will show
an illustrative pathway of the reaction of H![]() O
O![]() with Fe
with Fe![]() in water, which includes the coordination process.
in water, which includes the coordination process.


|