In this section, new reactive pathways for the O-O bond breaking upon
coordination of an hydrogen
peroxide molecule to an iron(II) ion in aqueous solution will be
generated, using the transition path sampling technique. To this end,
a point on an existing reaction pathway has to be chosen as the starting
configuration of a new pathway. In the present sampling procedure, a configuration
file with the atomic configurations and wavefunction coefficients was saved
every 1000 time steps (145 femtoseconds). One of the saved
configurations was randomly chosen as the starting point for a new
pathway. The first new successful reaction path initiated from a point on our
initial pathway, will be referred to as the first generation path;
a successful reaction path initiated from a point on this first
generation path makes than a second generation path, etcetera.
A sequence of reactive pathways is a series of subsequent
generations.
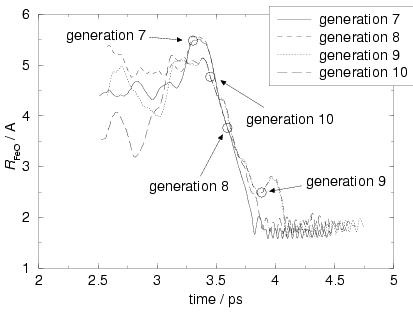
Figure 7.4 illustrates the procedure for four pathways;
the circles denote the chosen starting points where on each ![]() generation path a new
generation path a new
![]() generation path branches off.
On a certain pathway, the time
between its own starting point and the starting point of the next
pathway, varied between 0.29 and 0.58 picoseconds, which is
in a sense the time that the system is allowed to relax on a pathway before
a next generation one branches off.
generation path branches off.
On a certain pathway, the time
between its own starting point and the starting point of the next
pathway, varied between 0.29 and 0.58 picoseconds, which is
in a sense the time that the system is allowed to relax on a pathway before
a next generation one branches off.
|
We calculated two sequences (sequence A and sequence B),
each with a length of 10 generations. For the first generation of each sequence
we took one of the 20 trajectories calculated earlier to verify the transition
state location on the initial reaction pathway, shown in the right-hand-side
plots of figure 7.3. That is, the first generation
pathways for both sequences A and B branch off at the transition state
point of our initial pathway, with random atomic momenta drawn from a
![]() K Boltzmann distribution. Of course, figure 7.3
shows only halves of reactive pathways (namely from the TS point to
either the product state or the reactant state), so the other half was
calculated, for our two first generations, by integrating the equations of
motion backwards in time from the starting configuration of the
reactive pathway (i.e. the TS point).
K Boltzmann distribution. Of course, figure 7.3
shows only halves of reactive pathways (namely from the TS point to
either the product state or the reactant state), so the other half was
calculated, for our two first generations, by integrating the equations of
motion backwards in time from the starting configuration of the
reactive pathway (i.e. the TS point).
In table 7.2, which shows some characteristics
of the computed pathways for sequence A (first 11 rows) and sequence B
(last 9 rows), we see that the first generation pathways of sequences
A and B are quite different from each other. For sequence A, the pathway
does not show the direct mechanism of our initial pathway in which the
ferryl ion (Fe![]() O
O![]() ) is formed via a rebound of the leaving
OH. radical, which abstracts the hydrogen of the intermediate
Fe
) is formed via a rebound of the leaving
OH. radical, which abstracts the hydrogen of the intermediate
Fe![]() -OH. Instead, the leaving OH. radical jumps after
a lifetime of
-OH. Instead, the leaving OH. radical jumps after
a lifetime of
![]() fs via two solvent water
molecules and terminates at a water ligand of a periodic image of the
iron complex in a neighboring copy of the unit cell to form the dihydroxo
iron(IV) moiety. This is indeed the first step of the two-step mechanism that we
have seen before
in simulations starting from hydrogen peroxide already coordinated to
pentaaquairon(II).[171,146] In a second step (see last
column in table 7.2), the ferryl
ion was formed by donation of a proton by one of the hydroxo ligands to
the solvent. For sequence B, the first generation pathway shows the rebound
mechanism, although also an incipient OH. radical shunt via two solvent
water molecules is observed, but this OH.-shunt is not completed,
the motion of the solvent hydrogens is reversed when the Fe
fs via two solvent water
molecules and terminates at a water ligand of a periodic image of the
iron complex in a neighboring copy of the unit cell to form the dihydroxo
iron(IV) moiety. This is indeed the first step of the two-step mechanism that we
have seen before
in simulations starting from hydrogen peroxide already coordinated to
pentaaquairon(II).[171,146] In a second step (see last
column in table 7.2), the ferryl
ion was formed by donation of a proton by one of the hydroxo ligands to
the solvent. For sequence B, the first generation pathway shows the rebound
mechanism, although also an incipient OH. radical shunt via two solvent
water molecules is observed, but this OH.-shunt is not completed,
the motion of the solvent hydrogens is reversed when the Fe![]() -OH
intermediate donates its hydrogen to the leaving OH. radical.
-OH
intermediate donates its hydrogen to the leaving OH. radical.
From these two first generation pathways, we successively generated
new pathways by taking a configuration from a path and changing the
atomic momenta. The momenta were changed by randomly drawing new
momenta from a gaussian distribution of ![]() K or
K or ![]() K and adding these
to the old momenta (and correcting for any total momentum of the system).
The success rate of obtaining a new pathway connecting reactants and
products was about 50%. In both sequences, accidentally,
the fifth generation
pathway was started from an unsuccessful fourth generation
pathway that started in the product well and recrossed back to
the product well, although the iron-oxygen distances reached a
separation of more than 4 Å (which is our order parameter defining the
reactant well) in both fourth generation pathways.
K and adding these
to the old momenta (and correcting for any total momentum of the system).
The success rate of obtaining a new pathway connecting reactants and
products was about 50%. In both sequences, accidentally,
the fifth generation
pathway was started from an unsuccessful fourth generation
pathway that started in the product well and recrossed back to
the product well, although the iron-oxygen distances reached a
separation of more than 4 Å (which is our order parameter defining the
reactant well) in both fourth generation pathways.
|
|
|||||
| generation | mechanism |
|
# H |
terminating | final obs. |
| H-bond wire | Fe complex | species | |||
|
|
|||||
|
|
|||||
| Relaxation sequence A | |||||
| 1 | long wire | 149 | 2 | copy | ferryl |
| 2 | long wire | 380 | 2 | copy | ferryl |
| 3 | short wire | 322 | 0 | same | ferryl |
| 5 |
long wire | 289 | 2 | copy | Fe |
| 6 | long wire | 265 | 2 | copy | Fe |
| 7 | long wire | 150 | 3 | copy | ferryl |
| 7 | short wire | 70 | 1 | same | ferryl |
| 8 | short wire | 73 | 1 | same | Fe |
| 9 | short wire | 70 | 1 | same | Fe |
| 9 | short wire | 65 | 1 | same | Fe |
| 10 | short wire | 66 | 1 | same | Fe |
| generation | mechanism |
|
# H |
terminating | final obs. |
| H-bond wire | Fe complex | species | |||
|
|
|||||
|
|
|||||
| Relaxation sequence B | |||||
| 1 |
both | 101 | 2 | both | ferryl |
| 2 | direct | 121 | 0 | same | ferryl |
| 3 | direct | 133 | 0 | same | ferryl |
| 5 |
direct | 251 | 0 | same | ferryl |
| 6 | direct | 248 | 0 | same | ferryl |
| 7 | direct | 141 | 0 | same | ferryl |
| 8 | long wire | 43 | 2 | copy | Fe |
| 9 | long wire | 42 | 2 | copy | Fe |
| 10 | short wire | 93 | 1 | same | Fe |
Taking a closer look at table 7.2, we see trends along
the two sequences which could indicate that indeed our initial pathway
is an atypical one and relaxation towards more representative pathways
does take place. For example, the time that
the leaving OH. radical remains intact before abstracting a hydrogen
from the complex or a solvent water (the lifetime
![]() , which is measured from the moment of O-O
lysis, defined as
, which is measured from the moment of O-O
lysis, defined as
![]() Å, until the first H-abstraction
by OH.),
is seen to decrease in both sequences. Secondly, in both sequences
the followed mechanisms change via or from the ``long-wire two-step''
mechanism (in which the OH. radical in the first step terminates via a wire of
two or three solvent waters at a water ligand of the periodic image
of the complex) to the ``short-wire two step'' mechanism. In the
latter case, the leaving OH. radical terminates in the first step at an adjacent
water ligand (thus stays in the same unit cell) via one bridging
solvent molecule, forming the dihydroxo iron(IV) complex.
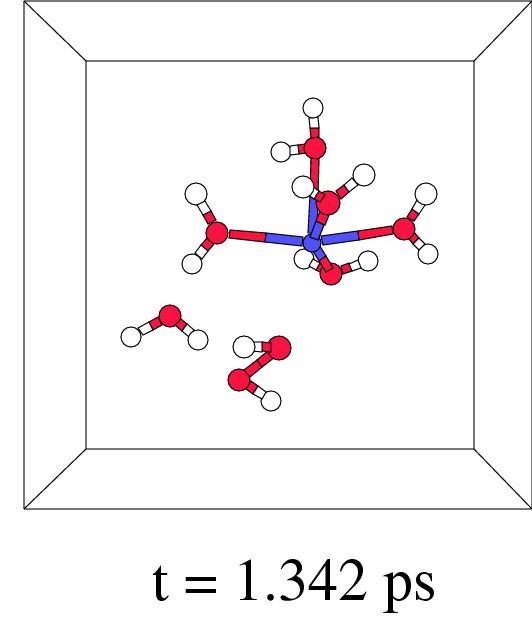
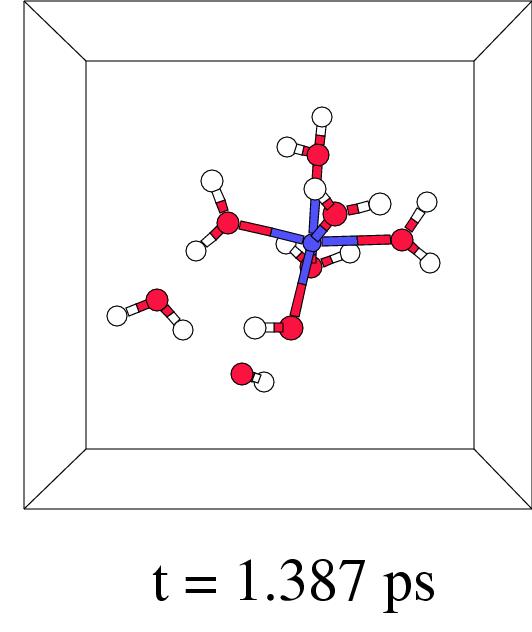
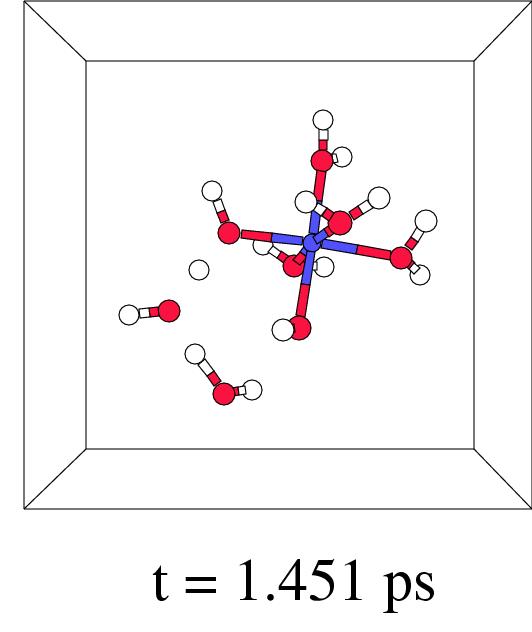
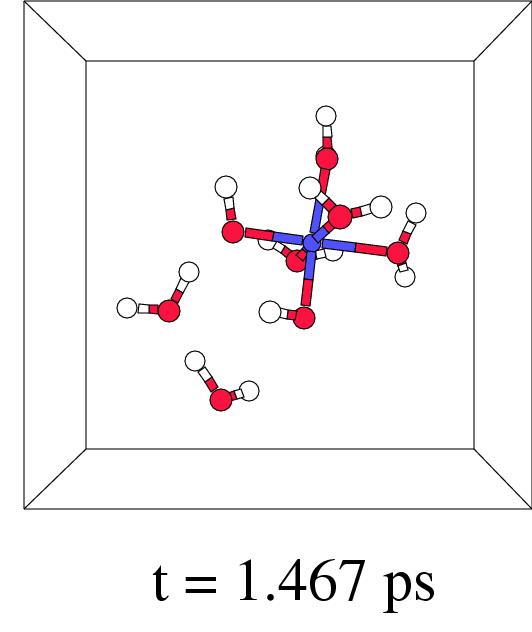
Figure 7.5 shows in four snapshots the H
Å, until the first H-abstraction
by OH.),
is seen to decrease in both sequences. Secondly, in both sequences
the followed mechanisms change via or from the ``long-wire two-step''
mechanism (in which the OH. radical in the first step terminates via a wire of
two or three solvent waters at a water ligand of the periodic image
of the complex) to the ``short-wire two step'' mechanism. In the
latter case, the leaving OH. radical terminates in the first step at an adjacent
water ligand (thus stays in the same unit cell) via one bridging
solvent molecule, forming the dihydroxo iron(IV) complex.
Figure 7.5 shows in four snapshots the H![]() O
O![]() coordination to iron(II), the O-O lysis, and the OH. radical
shunt and termination, of such a short-wire step. In fact,
this new ``short-wire'' reaction pathway was already predicted in
previous work where we discussed the possibilities for a radical
shunt in a very large box containing a single pentaaqua iron(II)
hydrogen peroxide complex.[171] In our present pathway
relaxation procedure it indeed appears spontaneously.
coordination to iron(II), the O-O lysis, and the OH. radical
shunt and termination, of such a short-wire step. In fact,
this new ``short-wire'' reaction pathway was already predicted in
previous work where we discussed the possibilities for a radical
shunt in a very large box containing a single pentaaqua iron(II)
hydrogen peroxide complex.[171] In our present pathway
relaxation procedure it indeed appears spontaneously.
|
The last column in table 7.2 displays the last observed
iron complex. It shows that
not always the ferryl ion is formed, but instead in many cases
an [Fe![]() (H
(H![]() O)
O)![]() (OH)
(OH)![]() ]
]![]() complex. This is
due to the dynamic equilibrium between the acidic dihydroxo species and
its conjugate base, the hydrolyzed trihydroxo species,
by proton donation to the solvent:
complex. This is
due to the dynamic equilibrium between the acidic dihydroxo species and
its conjugate base, the hydrolyzed trihydroxo species,
by proton donation to the solvent: