The system can be constrained to a hyperplane
![]() in phase space in a molecular simulation, by extending the Lagrangian
with a term
in phase space in a molecular simulation, by extending the Lagrangian
with a term
![]() , with
, with ![]() the atomic positions,
the atomic positions, ![]() our reaction coordinate, and
our reaction coordinate, and ![]() the
Lagrange multiplier associated with the force on the holonomic constraint.
Each atom
the
Lagrange multiplier associated with the force on the holonomic constraint.
Each atom ![]() then feels a constraint force, equal to
then feels a constraint force, equal to
![]() .
It is well known that the use of constraints affects the phase space
distribution (see e.g. ref frenkel_smit).
For a velocity-independent property
.
It is well known that the use of constraints affects the phase space
distribution (see e.g. ref frenkel_smit).
For a velocity-independent property ![]() , the bias introduced by a
constraint can be compensated using the relation
, the bias introduced by a
constraint can be compensated using the relation
where the factor ![]() is defined by
is defined by
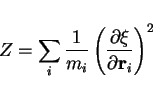 |
(52) |
This was generalized recently for velocity dependent properties, such as the the mean force of constraint[17,76]. In the formulation of reference otterw3 it reads:
where ![]() is the temperature,
is the temperature, ![]() is Boltzmann's constant, and
is Boltzmann's constant, and
![]() is the mass of particle
is the mass of particle ![]() .
.
The second term in the numerator of equation 3.7
arises because the mean force of constraint depends on the velocities,
through the kinetic term
![]() in the force of constraint
(equation 1). In their work, they demonstrated the
importance of the corrections for a constrained bending angle of a tri-atomic
molecule and for a constrained dihedral angle in a tetra atomic molecule.
in the force of constraint
(equation 1). In their work, they demonstrated the
importance of the corrections for a constrained bending angle of a tri-atomic
molecule and for a constrained dihedral angle in a tetra atomic molecule.
In table 3.8, we show the results for the mean force
of constraint for our S![]() 2 reaction using the constraint of equation 2,
once as
2 reaction using the constraint of equation 2,
once as ![]() , once corrected according to equation 3.5
and once using equation 3.7. As the differences are very small,
the bias on our system introduced by the constraint must by very small.
, once corrected according to equation 3.5
and once using equation 3.7. As the differences are very small,
the bias on our system introduced by the constraint must by very small.
|
|
||||
|
|
||||
|
|
||||
| 0.27 | -0.0091 | -0.0098 | -0.0095 | |
|
|
||||
| 0.32 | -0.0494 | -0.0498 | -0.0496 | |
|
|
||||
| 0.35 | -0.0815 | -0.0831 | -0.0830 | |
|
|
||||
| 0.40 | -0.2384 | -0.2396 | -0.2394 | |
|
|
||||
| 0.43 | -0.3268 | -0.3277 | -0.3276 | |
|
|
||||
| 0.45 | -0.2929 | -0.2931 | -0.2930 | |
|
|
||||
| 0.50 | -0.0408 | -0.0408 | -0.0408 | |
|
|
||||
| 0.55 | 0.2570 | 0.2572 | 0.2571 | |
|
|
||||
| 0.60 | 0.1955 | 0.1966 | 0.1964 | |
|
|
||||
| 0.70 | 0.0018 | 0.0004 | 0.0002 |