

Next: Theoretical chemistry
Up: Introduction
Previous: Introduction
Contents
Water is one of the most plentiful and essential of compounds, which is vital
to life, participating in virtually every process that occurs in plants and animals.
In fact, life is believed to have originated in the worlds complex brews, the
oceans.
It is no wonder, that due to its prominence, water has long played
an important religious and philosophical role in human history. The Chinese
alchemist Tsou Yen (3 century BC) placed water among the five divine
elements (together with fire, earth, wood and metal) that make up the universe.
And the Greek Thales of Miletus (6
century BC) placed water among the five divine
elements (together with fire, earth, wood and metal) that make up the universe.
And the Greek Thales of Miletus (6 century BC) regarded water as the
sole fundamental building block of matter:
century BC) regarded water as the
sole fundamental building block of matter:
It is water that, in taking different forms, constitutes the earth, atmosphere,
sky, mountains, gods and men, beast and birds, grass and trees, and animals
down to worms, flies and ants. All these are different forms of water. Meditate
on water!
The great Aristotle included water among the four elements alongside earth,
air and fire, which belief persisted for more than 2000 years until experiments
in the second half of the 18 century showed that water is a compound
made up of the elements hydrogen and oxygen.
Unfortunately, nowadays practically none of us sees anymore the magic in such things as
our planet being covered with oceans of liquid water, in solid rocks of ice floating
on water, in sugar cubes disappearing in water whereas milk blends with
water (not to mention the behavior of tea leaves in water), in clouds of water
flying in the air or even in its beautiful appearance in a rainbow and in the
water crystals in snowflakes.
Yet, especially in the eyes of physicists and chemists, water is an extraordinary
substance with very unique properties. For example, its boiling point of
100
century showed that water is a compound
made up of the elements hydrogen and oxygen.
Unfortunately, nowadays practically none of us sees anymore the magic in such things as
our planet being covered with oceans of liquid water, in solid rocks of ice floating
on water, in sugar cubes disappearing in water whereas milk blends with
water (not to mention the behavior of tea leaves in water), in clouds of water
flying in the air or even in its beautiful appearance in a rainbow and in the
water crystals in snowflakes.
Yet, especially in the eyes of physicists and chemists, water is an extraordinary
substance with very unique properties. For example, its boiling point of
100 C is nearly 200
C is nearly 200 C higher than would be expected by comparison
with other molecules as small as the water molecule.
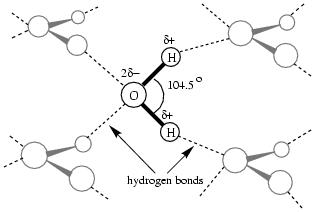
The secret behind the unexpected properties of water lies in the electronic structure
of the oxygen atom and the two hydrogen atoms which make up the bent water molecule
(H
C higher than would be expected by comparison
with other molecules as small as the water molecule.
The secret behind the unexpected properties of water lies in the electronic structure
of the oxygen atom and the two hydrogen atoms which make up the bent water molecule
(H O), as shown in figure 1.1. It causes the hydrogens to
carry each a positive charge of about one third of an electron and the oxygen
a negative charge of about two thirds on an electron, inducing a significant
dipole moment
in the water molecule. The electronegative oxygen atom has two non-bonding electron
pairs that attract hydrogens from adjacent water molecules, so that the water
molecule associates strongly. The weak bonds that are this way formed between them,
are called hydrogen bonds,
which are also known to be responsible for the double helix
shape of DNA, the working of soap, and the perfect mixing of ethanol
and water, giving rise to the best mindboggling beverages known to man.
In an ice crystal, the water molecules are tetrahedrally surrounded by neighbors
and form an open highly ordered three-dimensional network of hydrogen bonds.
The formed tetrahedrons give rise to puckered hexagonal rings of water molecules,
which are responsible for the hexagonal symmetry of the ice crystals, e.g. in snowflakes. When the ice melts, the highly order structure breaks down and
the molecules pack more closely together. This makes liquid water denser than
ice which is why the solid floats on the liquid; another highly unusual property
of water. However, also in the liquid, hydrogen bonding remains the important
associative force, responsible for the unusually high values for the boiling point,
the surface tension, the heat and entropy of vaporization and the viscosity,
given the low molar mass of H
O), as shown in figure 1.1. It causes the hydrogens to
carry each a positive charge of about one third of an electron and the oxygen
a negative charge of about two thirds on an electron, inducing a significant
dipole moment
in the water molecule. The electronegative oxygen atom has two non-bonding electron
pairs that attract hydrogens from adjacent water molecules, so that the water
molecule associates strongly. The weak bonds that are this way formed between them,
are called hydrogen bonds,
which are also known to be responsible for the double helix
shape of DNA, the working of soap, and the perfect mixing of ethanol
and water, giving rise to the best mindboggling beverages known to man.
In an ice crystal, the water molecules are tetrahedrally surrounded by neighbors
and form an open highly ordered three-dimensional network of hydrogen bonds.
The formed tetrahedrons give rise to puckered hexagonal rings of water molecules,
which are responsible for the hexagonal symmetry of the ice crystals, e.g. in snowflakes. When the ice melts, the highly order structure breaks down and
the molecules pack more closely together. This makes liquid water denser than
ice which is why the solid floats on the liquid; another highly unusual property
of water. However, also in the liquid, hydrogen bonding remains the important
associative force, responsible for the unusually high values for the boiling point,
the surface tension, the heat and entropy of vaporization and the viscosity,
given the low molar mass of H O.
O.
Figure 1.1:
In liquid and solid water (ice), the H O molecules form
so-called hydrogen bonds between the partially positive hydrogen atoms (H) and
partially negative oxygen atoms (O), forming a relatively strong tetrahedral
network.
O molecules form
so-called hydrogen bonds between the partially positive hydrogen atoms (H) and
partially negative oxygen atoms (O), forming a relatively strong tetrahedral
network.
|
|
One of the most important properties of water is its ability to dissolve ionic
and polar chemicals to form aqueous solutions. From ponts till oceans,
the water masses contain a vast number of dissolved substances, which are vital
to life. Moreover, in living organisms, water enables the transport of nutrients
and trace elements. The solvation of compounds involves complex rearrangements
in the solvent environment of the compound (i.e. the solvation shell),
in which again the polar nature of the water molecule and the hydrogen bond making
and breaking play the essential roles.
The structure and dynamics in these solvation shells are
closely connected to the way in which chemical reactions in water take place.
For instance, many reactions only occur after water molecules have been removed
from the solvation shells. Other examples are acid catalyzed reactions and radical
reactions in aqueous solution, in which respectively protons and hydroxyl radicals
rapidly move along hydrogen bond wires through the solvent by passing on hydrogens
from water molecule to molecule. Still, the dynamic solvent effects and the versatile
role of water in chemical reactions are poorly understood on a microscopic level,
which complicates the understanding of biological processes and slows down the
development of environmentally friendly industrial applications.
This thesis is perhaps not likely to bring back any of the magic around the special
behavior of water, but, no doubt, insight and understanding is gained of its involvement
in chemistry.



Next: Theoretical chemistry
Up: Introduction
Previous: Introduction
Contents
Bernd Ensing
2003-06-13
![]() century BC) placed water among the five divine
elements (together with fire, earth, wood and metal) that make up the universe.
And the Greek Thales of Miletus (6
century BC) placed water among the five divine
elements (together with fire, earth, wood and metal) that make up the universe.
And the Greek Thales of Miletus (6![]() century BC) regarded water as the
sole fundamental building block of matter:
century BC) regarded water as the
sole fundamental building block of matter: