


Next: About the author
Up: Chemistry in Water First
Previous: Dankwoord
Contents
- Methane oxidation by the ferryl ion
B. Ensing, F. Buda, and E. J. Baerends.
submitted for publication.
- O
 evolution in the Fenton reaction
evolution in the Fenton reaction
F. Buda, B. Ensing, M. C. M. Gribnau, and E. J. Baerends.
submitted for publication.
- Fenton-like chemistry in water:
oxidation catalysis by Fe(III) and H
 O
O
B. Ensing, F. Buda, and E. J. Baerends.
submitted for publication.
- Reaction path sampling of the reaction between iron(II) and hydrogen
peroxide in aqueous solution
B. Ensing and E. J. Baerends.
J. Phys. Chem. A 106, 7902-7910 (2002).
- A Car-Parrinello study of the formation of oxidizing intermediates
from Fenton's reagent in aqueous solution
B. Ensing, F. Buda, P. E. Blöchl, and E. J. Baerends.
Phys. Chem. Chem. Phys. 4, 3619-3627 (2002).
- Chemical involvement of solvent water molecules in elementary steps
of the Fenton reaction
B. Ensing, F. Buda, P. E. Blöchl, and E. J. Baerends.
Angew. Chem. Int. Edit. 40, 2893-2895 (2001).
- DFT studie of the active intermediate in the Fenton reaction
F. Buda, B. Ensing, M. C. M. Gribnau, and E. J. Baerends.
Chem. Eur. J. 7, 2775-2783 (2001).
- Solvation effects on the S
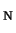 2 reaction between
CH
2 reaction between
CH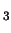 Cl + Cl
Cl + Cl in water
in water
B. Ensing, E. J. Meijer, P. E. Blöchl, and E. J. Baerends.
J. Phys. Chem. A 105, 3300-3310 (2001).
- Comparison of the accurate Kohn-Sham solution with the generalized gradient
approximations (GGAs) for the S
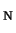 2 reaction, F
2 reaction, F + CH
+ CH F
F
 FCH
FCH + F
+ F : A qualitative rule to predict success or
failure of GGAs
: A qualitative rule to predict success or
failure of GGAs
O. V. Gritsenko, B. Ensing, P. R. T. Schipper, and E. J. Baerends.
J. Phys. Chem. A 104, 8558-8565 (2000).
- A combined molecular dynamics ab initio study of H
 adsorption on
ideal, relaxed, and temperature-reconstructed MgO(111) surfaces
adsorption on
ideal, relaxed, and temperature-reconstructed MgO(111) surfaces
K. Hermansson, M. Baudin, B. Ensing, M. Alfredsson, and M. Wojcik.
J. Phys. Chem. 109, 7515-7521 (1998).



Next: About the author
Up: Chemistry in Water First
Previous: Dankwoord
Contents
Bernd Ensing
2003-06-13